Usable data
↑
With harmonized pipelines
Device onboarding
Fast
BYOD or provisioned + SIM
Signal to insight
Near-real-time
QC + alerts + workflows
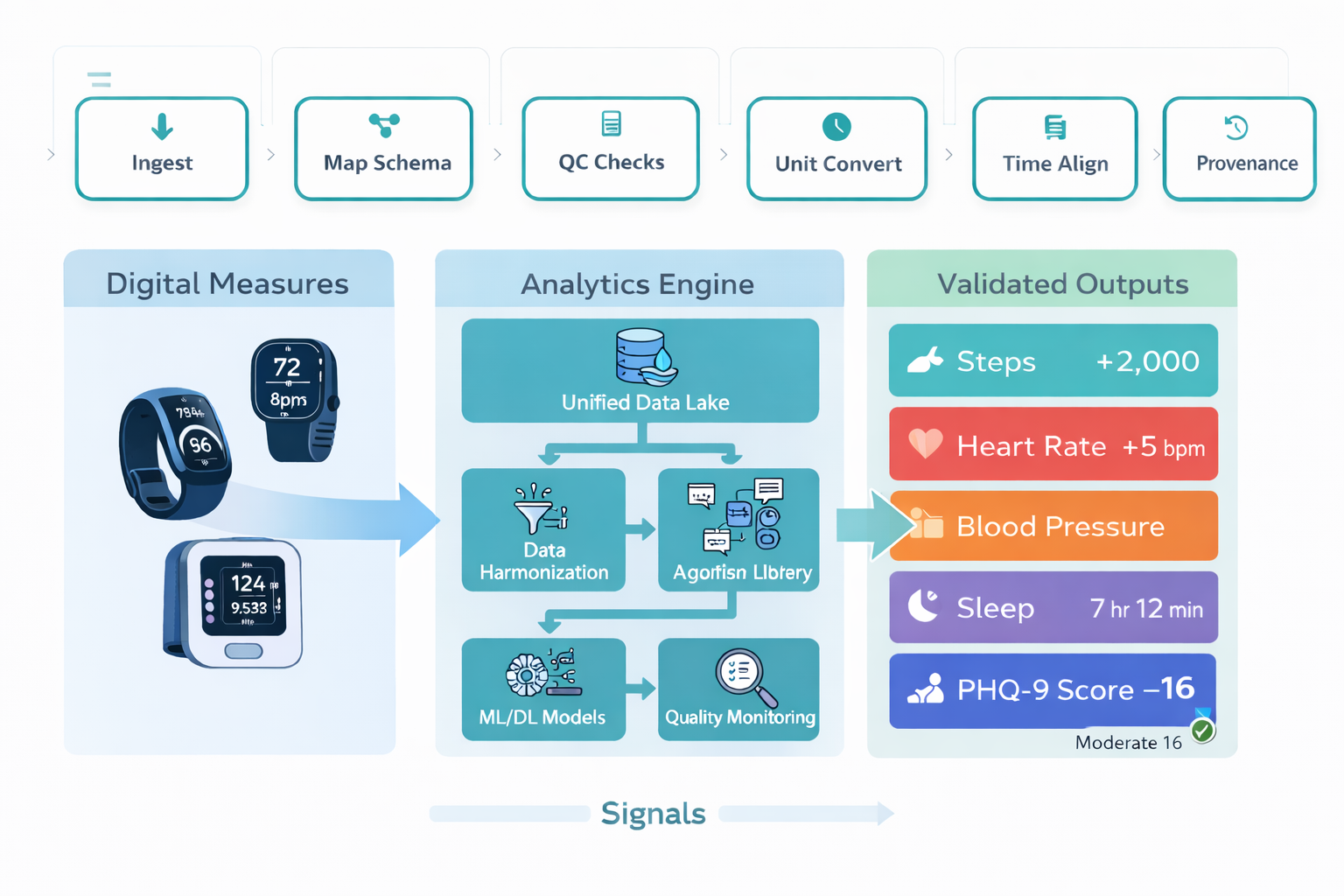
Digital Measures Pipeline
Device → QC → Harmonize → Endpoint
From Sensors to Submissions
Sponsors don’t just need wearables—they need digital measures designed for accuracy, reproducibility, and clinical relevance.
Delve converts multi-device signals into standardized metrics and validated digital endpoints, aligned to study definitions and operational reality across hybrid and decentralized trials.
- Fit-for-purpose digital endpoint development
- Correlation to gold standards
- Algorithm training & verification pathways
- Regulatory-aligned study documentation & traceability
Wearable Modalities Supported
Fit-for-purpose device strategies aligned to endpoint sensitivity, patient burden, and operational feasibility.
Activity & Mobility
Steps, gait proxies, activity tolerance, functional change.
Cardiovascular
HR, HRV, ECG (device-dependent), blood pressure.
Respiratory
SpO₂, respiratory rate, symptom-linked physiology.
Sleep
Duration, efficiency, staging where supported.
Metabolic
CGM, weight scales, longitudinal trend analysis.
Temperature & Vitals
Skin temperature and longitudinal vital trends.
Device Integration & Signal Harmonization
Connect any wearable or sensor, harmonize signals into a consistent clinical model, and keep data clean with automated QC.
Universal Device Compatibility
Connect medical wearables, consumer sensors, cellular devices, or BYOD—all in one platform.
Real-Time Signal Harmonization
Normalize HR, HRV, SpO₂, sleep, steps, and respiratory data into a consistent clinical data model.
Automated QC & Signal Integrity
Detect outliers, missing samples, device drift, timestamp errors, and physiological anomalies.
Open Endpoint APIs
Export harmonized endpoints directly to EDC, analytics pipelines, or regulatory submission packages.
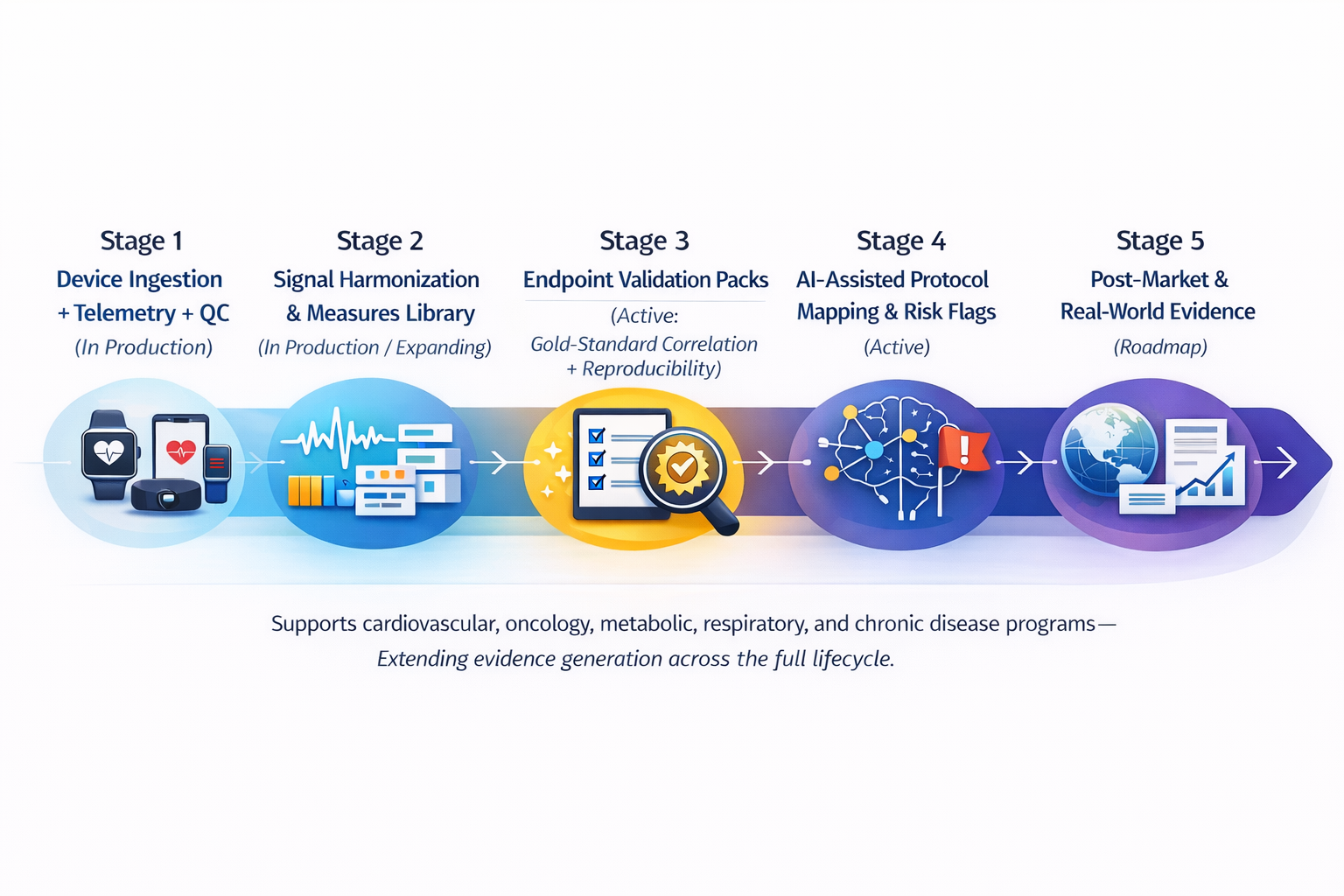
Digital Endpoint R&D Pipeline
Delve is building a defensible pipeline for digital endpoints and AI-enabled study operations—focused on making sensor data submission-grade and operationally reliable in the real world.
- Stage 1: Device ingestion + telemetry + QC (in production)
- Stage 2: Signal harmonization and measures library (in production / expanding)
- Stage 3: Endpoint validation packs (active: gold-standard correlation + reproducibility)
- Stage 4: AI-assisted protocol mapping & risk flags (active)
- Stage 5: Post-market and real-world evidence workflows (roadmap)
This approach supports cardiovascular, oncology, metabolic, respiratory, and chronic disease programs— and extends evidence generation across the full lifecycle.
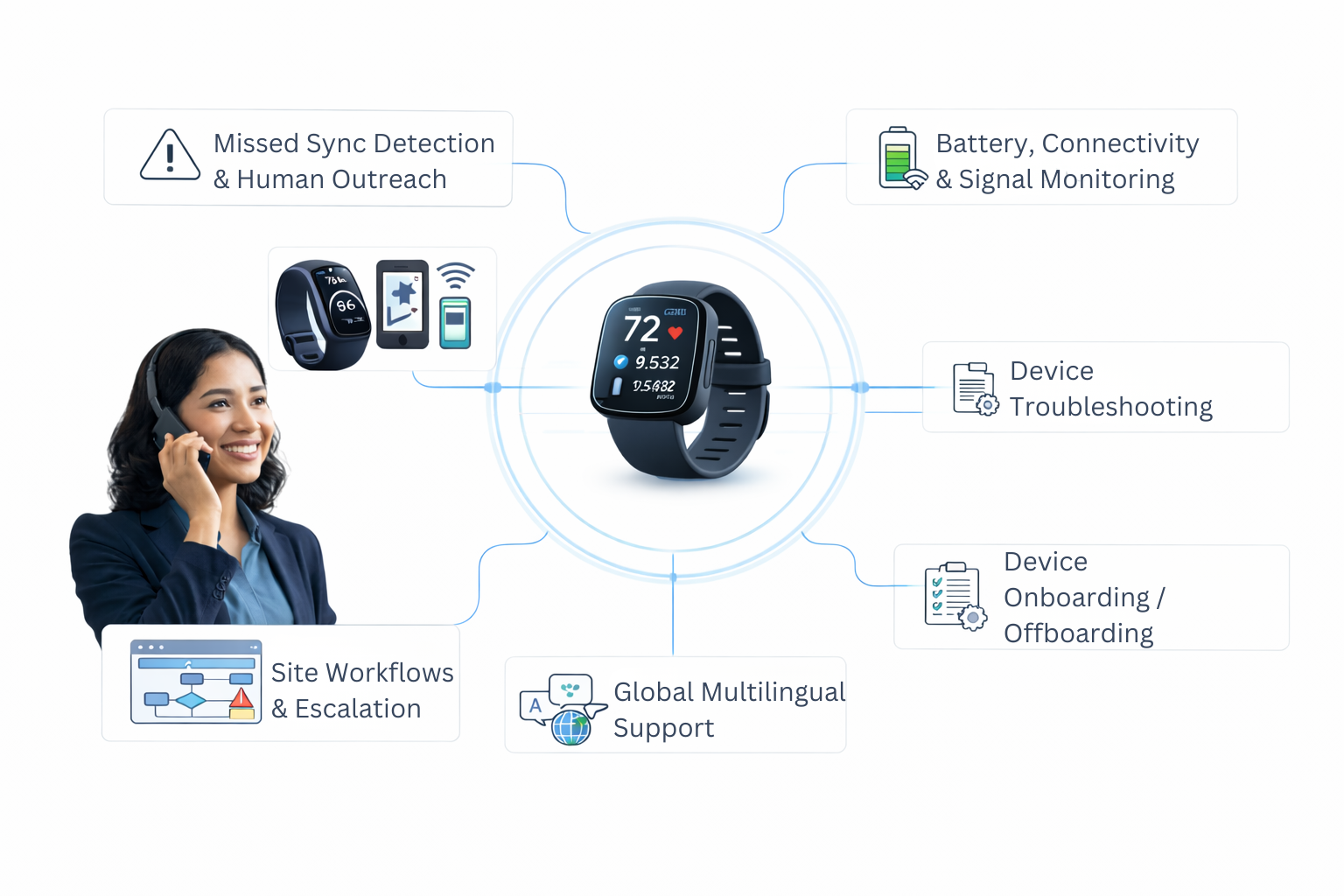
The Wearables Reality No One Talks About
Digital measures fail not because the sensors fail— but because no one operationalizes the devices.
Patients forget to charge devices. Sites are overwhelmed. CROs lack playbooks. Data gaps grow quietly.
Delve steps in with automation + real human follow-up so every wearable actually works in the real world.
- Missed sync detection & human outreach
- Battery, connectivity & signal monitoring
- Device troubleshooting & onboarding
- Site workflows & escalation paths
- Global multilingual support
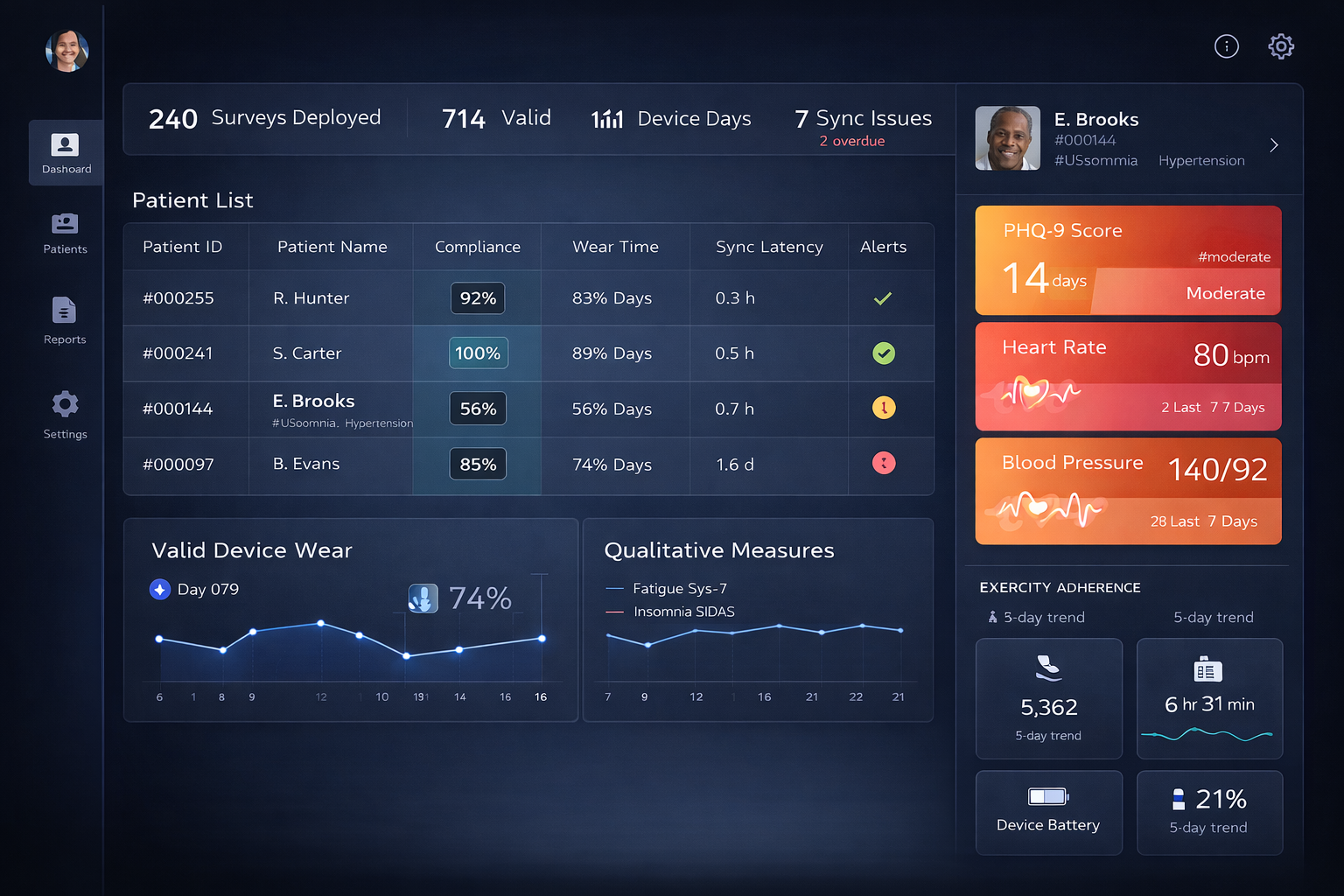
Digital Endpoints & Real-Time Clinical Insights
Transform raw sensor data into validated digital endpoints with live visibility into patient status, signal quality, and clinical trends.
Validated Algorithms
Gait speed, step quality, HR/HRV, sleep staging, SpO₂ trends, respiratory rate, and more.
Regulatory-Ready Digital Endpoints
Developed using correlation, training, verification, and FDA-aligned methodologies.
Live Endpoint Dashboards
Monitor patient status, signal stability, algorithm outputs, and longitudinal trends.
Endpoint + ePRO Fusion
Combine symptoms, diaries, and objective measures into unified digital outcomes.
Unified Clinical Dashboard
Endpoints, ePRO, and wearable signals combined into one clinical command center.
Engagement & Compliance Engine
Compliance is not a reminder problem. It’s an operational one. Delve combines automation with human intervention to keep studies on track—continuously.
Human Concierge Support
Onboarding, troubleshooting, reminders, and missed sync recovery.
Automated Issue Detection
Hardware failures, low battery, connectivity loss—caught instantly.
Closed-Loop Compliance
Automated triggers plus human outreach ensure continuous evidence collection.
95%+ Compliance
Industry-leading adherence across global, multi-device clinical studies.
Validation, Deployment & Global Scale
Digital measures only matter if they are defensible, deployable, and supported at scale. Delve operationalizes validation, global device logistics, and around-the-clock study support.
FDA/EMA Aligned
Fit-for-purpose validation and reproducibility checks for digital submissions.
Provisioning & Logistics
Shipping, SIM activation, returns, replacements, and inventory management.
BYOD + Medical-Grade
Support for both patient-owned and sponsor-provided wearables.
24/7 Global Support
Technical, patient-facing, and site support teams available worldwide.
See How Digital Measures Transform Your Study
Schedule a live walkthrough of the unified platform powering sensors, endpoints, analytics, and engagement.
Book a Demo