Endpoint readiness
Protocol-aligned
Definitions + outputs standardized
Quality controls
Real-time
Missingness, outliers, device drift
Traceability
Auditable
From raw signal → endpoint
Endpoint Pipeline
Signal → QC → Validation → Endpoint
From Raw Signals to Regulator-Ready Evidence
Wearable data is noisy, inconsistent, and device-dependent. Digital biomarkers solve this by applying validated algorithms, statistical models, and context-aware transformations that turn continuous sensor data into meaningful, clinical-grade measurements.
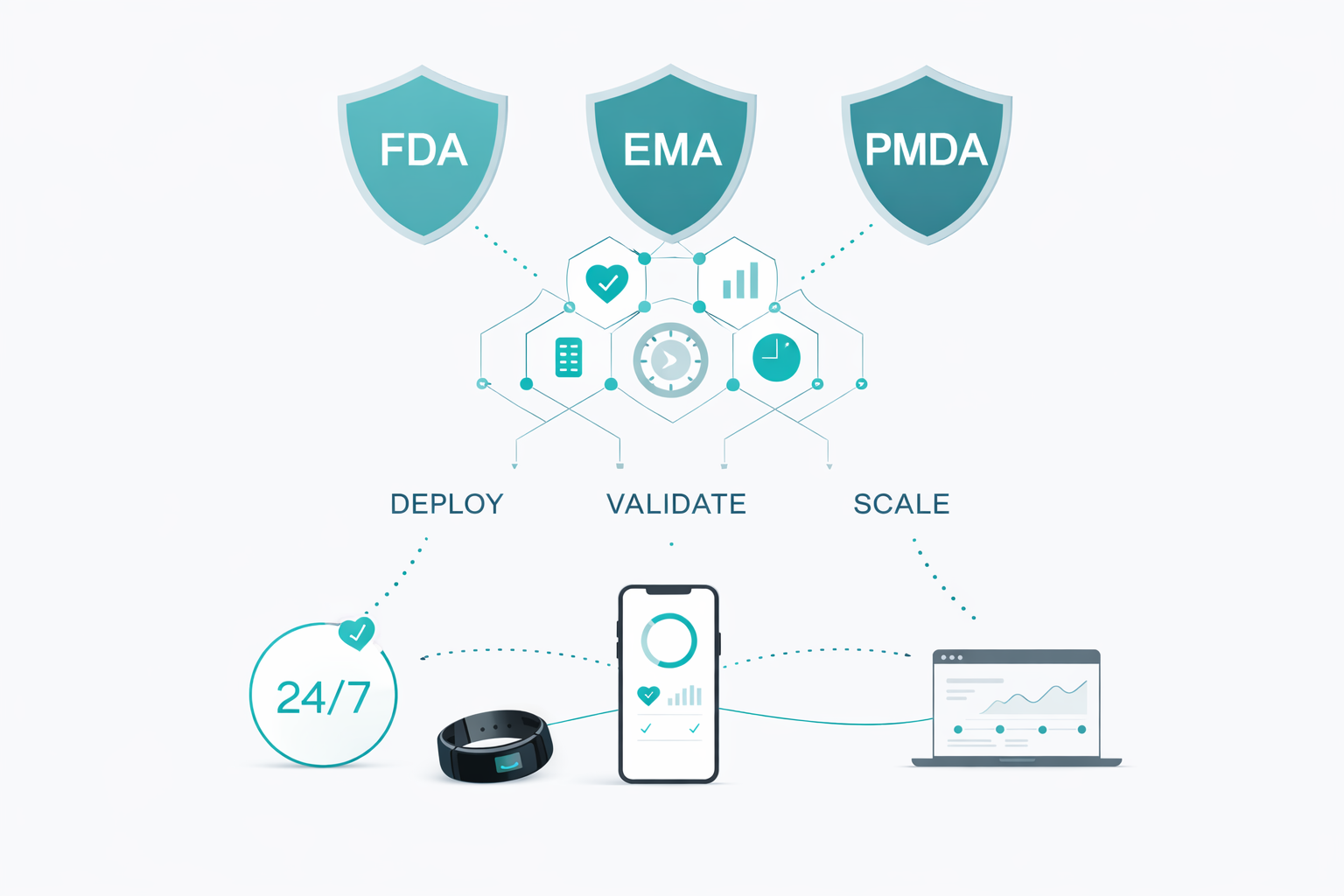
Delve enables sponsors to rapidly deploy, validate, and scale digital biomarkers aligned with FDA, EMA, and PMDA digital health technology frameworks.
- Fit-for-purpose validation for clinical use
- Algorithm transparency & reproducibility
- Cross-device measurement equivalence
- Science-driven QC & accuracy checks
- End-to-end endpoint management
Our 3-Phase Digital Endpoint Validation Framework
A regulator-aligned, stepwise approach to transforming raw sensor signals into defensible, submission-ready digital endpoints.
Correlation & Feasibility
Compare sensor-derived signals against gold-standard clinical measures to establish feasibility and relevance.
Algorithm Training & Calibration
Build reproducible, device-agnostic algorithms with controlled sampling, normalization, and signal quality checks.
Verification & Submission Readiness
Accuracy, variance, reproducibility, and context-of-use validation aligned to FDA and EMA expectations.
Digital Biomarker Library
Delve supports a broad and continuously expanding catalog of digital biomarkers. Each is validated for physiological accuracy, sampling reliability, and clinical interpretability.
Cardiovascular
Respiratory
Activity & Function
Sleep & Circadian
Subjective + Objective = True Digital Endpoints
The future of endpoints is hybrid. Delve fuses wearable signals with ePRO triggers, symptom diaries, and clinician assessments to produce composite endpoints with richer explanatory power.
- Sensor-driven ePRO triggers
- Event-linked symptom capture
- Composite endpoints blending function + perception
- Automated outlier detection
- Longitudinal trend modeling
This is how Delve reveals the story behind every datapoint — the human experience inside the physiology.
Digital Biomarkers in Action
Cardio-Oncology Study
Real-time HRV + symptom correlation predicted early toxicity signals.
Respiratory Trial
Nocturnal respiratory patterns improved early exacerbation detection.
Mobility Study
Gait speed and 6MWT digital equivalents aligned with clinician assessments.
Explore Delve’s Digital Biomarker Portfolio
See how validated digital endpoints can accelerate your next study.
Book a Demo