Risk flagged early
< 24h
From first signal
Data latency
Near-real-time
Wearables + ePRO + endpoints
Query pressure
↓
With automated QC rules
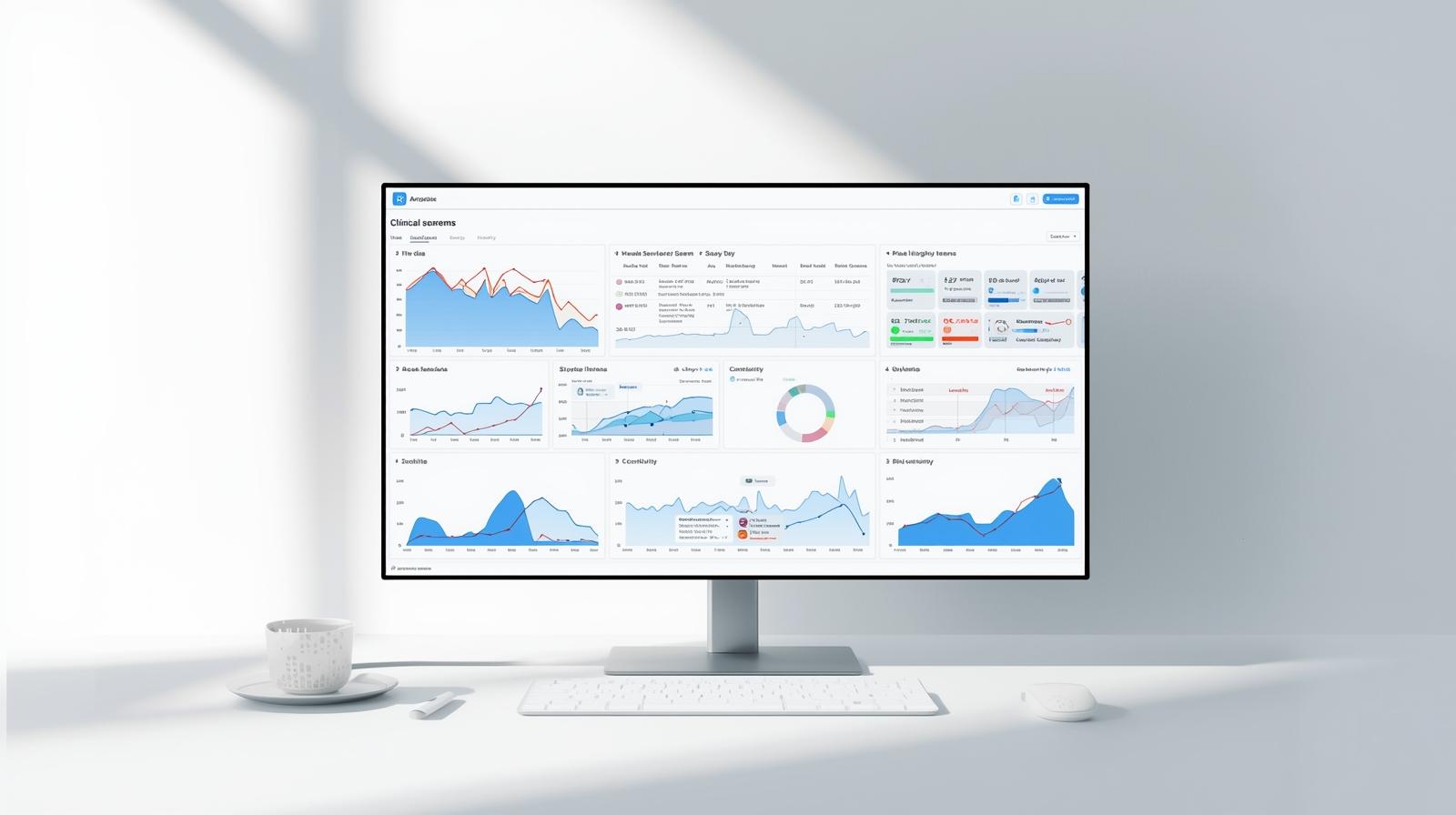
Study Health Dashboard
Live signals · Cross-source monitoring
One Dashboard. Every Signal.
Delve Analytics brings together wearable sensor streams, ePRO responses, device health, compliance, and digital biomarkers into a single, real-time platform built for modern research.
- Role-based dashboards for sites, CRAs, sponsors & data science teams
- Instant access to patient status, compliance, and data quality
- Device monitoring: battery, connectivity, sync health
- Wearable-derived biomarkers and trend modeling
- Immediate alerts when a patient falls behind
No more waiting weeks for vendor exports — evidence streams in continuously.
The Unified Delve Analytics Dashboard
Adaptively shows only the studies, sites, and patients tied to your role.
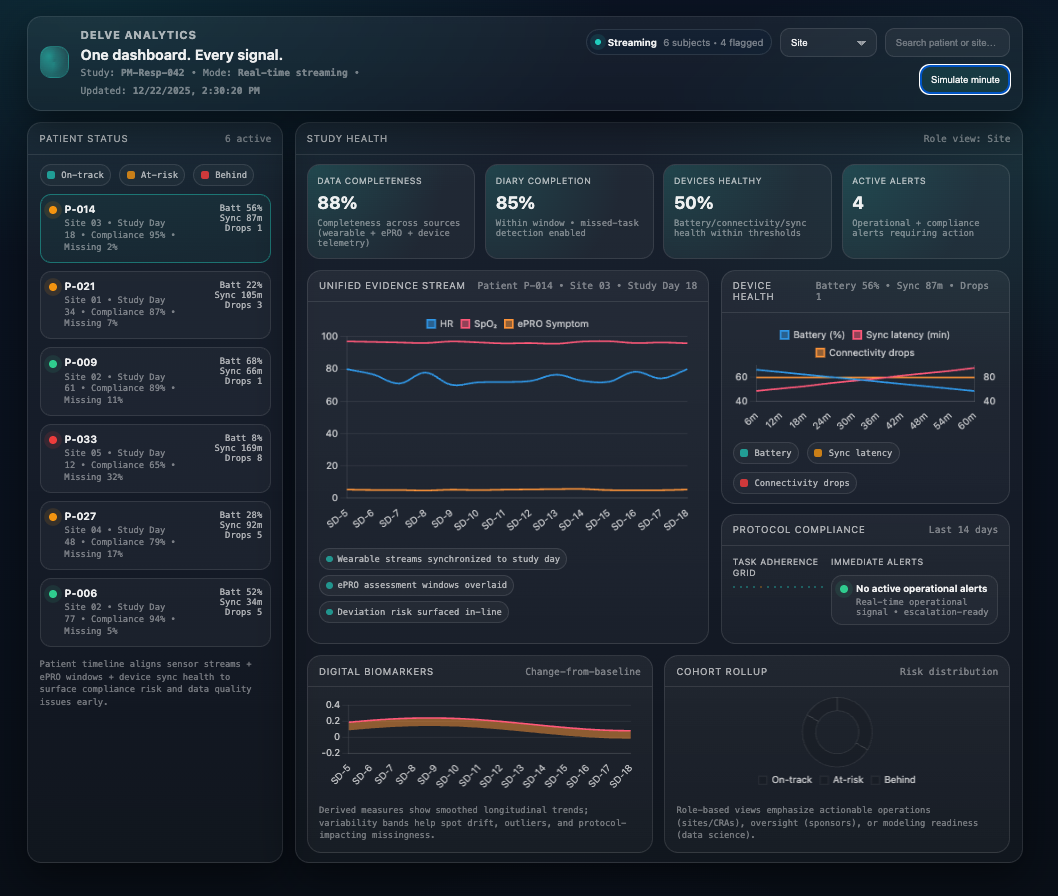
The Four Pillars of Delve Analytics
Study Oversight
Enrollment, randomization, study flow, deviations, missing tasks, and real-time quality metrics.
Patient-Level Insights
Sensor trends, PRO history, compliance curves, symptom patterns, and physiological changes — updated continuously.
Device Monitoring
Battery health, connectivity, missed syncs, wear-time estimation, device replacements, and troubleshooting flows.
Digital Endpoints
Wearable-derived biomarkers such as sleep, activity, HRV, gait, respiratory trends, and functional capacity indicators.
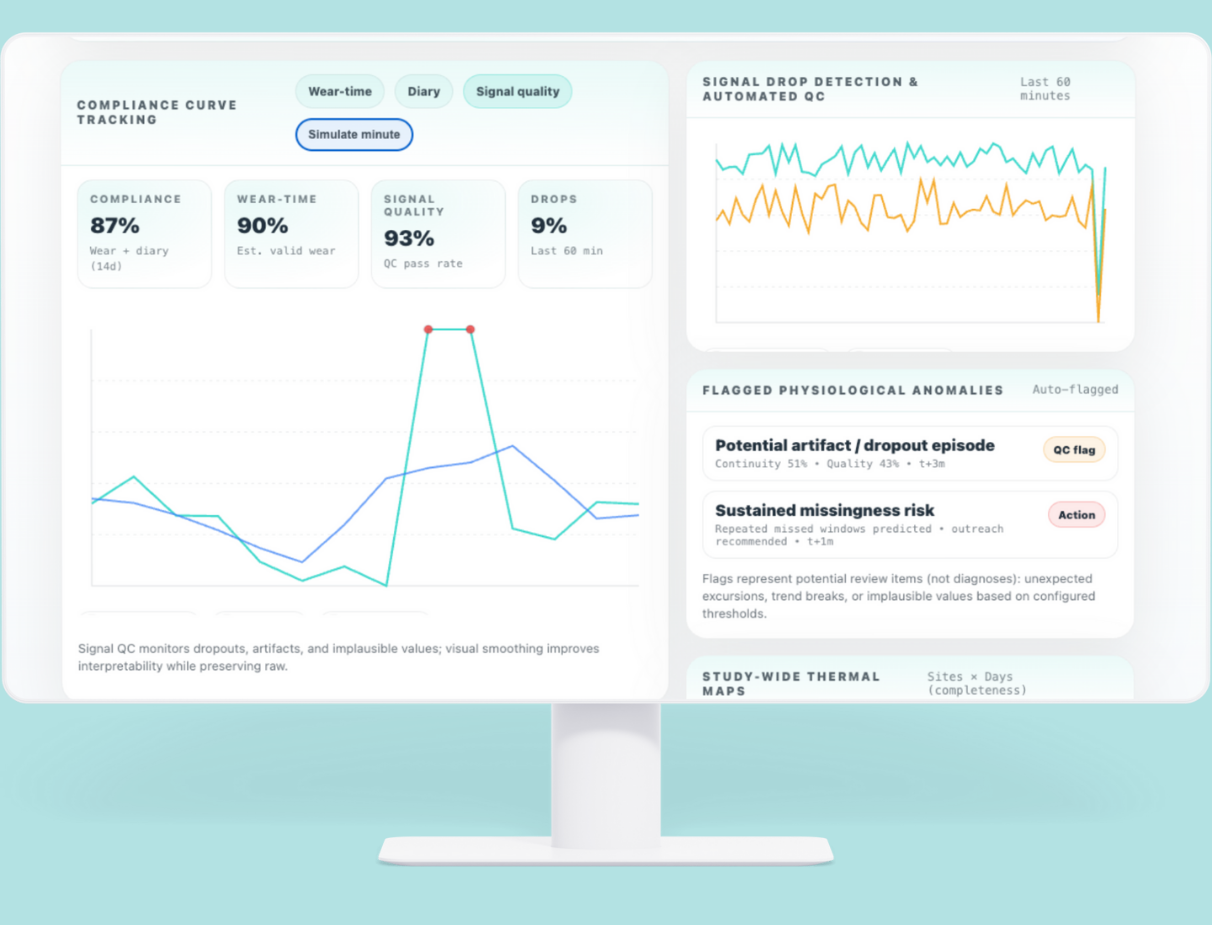
Compliance, Data Quality & Signal Health
Continuous research requires continuous insight. Delve monitors the full chain of evidence — from device to dashboard.
- Compliance curve tracking
- Wear-time estimation and accuracy
- Signal drop detection and automated QC
- Outlier removal and smoothing
- Flagged physiological anomalies
- Study-wide thermal maps
You see data the moment it changes — not weeks later.
Designed for Every Role in Research
Sponsors
Portfolio-level dashboards, risk detection, and early signals.
CRAs / MONs
Visit windows, missing tasks, and actionable site alerts.
Research Sites
Patient-level insights, onboarding status, and device troubleshooting.
Data Science Teams
Raw data access, algorithm outputs, and endpoint evaluations.
See Your Study in Real Time.
The dashboard designed for modern hybrid and decentralized research.
Book a Demo