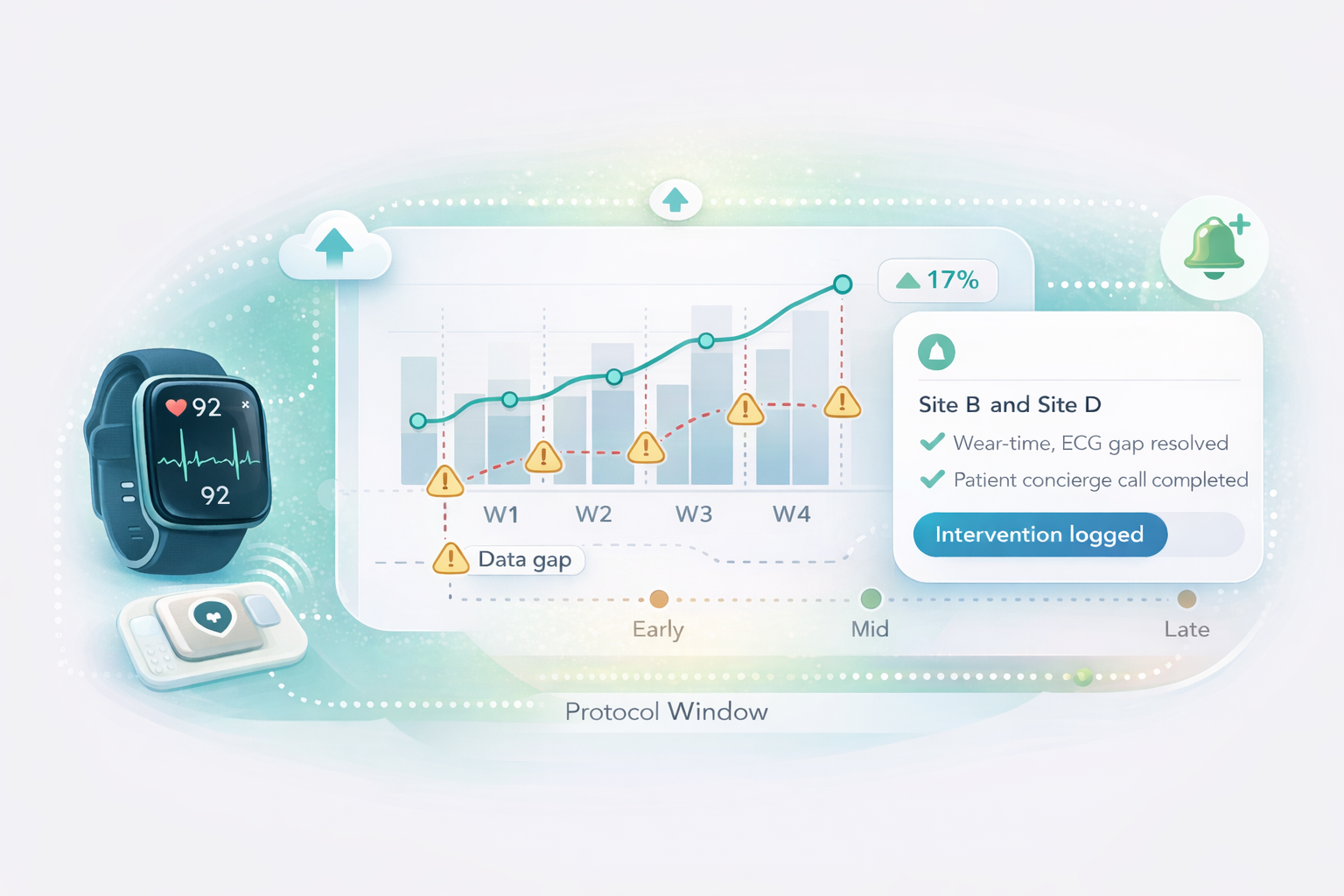
Signal QC & continuity monitoring
Quality gates for ECG/wearables, uploads, and data gaps—plus rescue workflows within protocol windows.
Cardiovascular Trials
Cardiovascular studies fail quietly: wear-time drops, ECG quality drifts, diaries go stale, and the “story” gets reconstructed at lock. Delve runs the between-visit operating model—real-time oversight + human follow-through—so missingness gets resolved early, not explained later.
Whether you’re measuring arrhythmia burden, BP control, functional capacity, symptoms, or post-market outcomes, Delve operationalizes protocol-driven routines with eCOA/ePRO, wearable and ECG workflows, logistics, and concierge support—so you get usable evidence, not just collected signals.
Wear-time continuity
Owned
Training + proactive rescue
Signal quality
Live
ECG/QC + gap alerts
Retention risk
Lower
Concierge absorbs follow-up
Designed for cardiovascular realities
Cardiovascular programs depend on high-fidelity longitudinal data. Delve enforces wear-time, validates signal quality, and rescues gaps before they become analysis risk.

Quality gates for ECG/wearables, uploads, and data gaps—plus rescue workflows within protocol windows.

Medication logs, BP routines, symptom reporting, and device habits reinforced by reminders and human follow-through.

Concierge handles training, troubleshooting, rescheduling, and device support—so sites focus on clinical assessments.
Long-term cardiovascular follow-up
Cardiovascular endpoints mature over months and years. As visit cadence tapers, traditional trial infrastructure fades — and participants drift without anyone truly owning continuity. Delve is built to keep the evidence chain intact between visits, when drop-off risk is highest and recovery windows close fast.
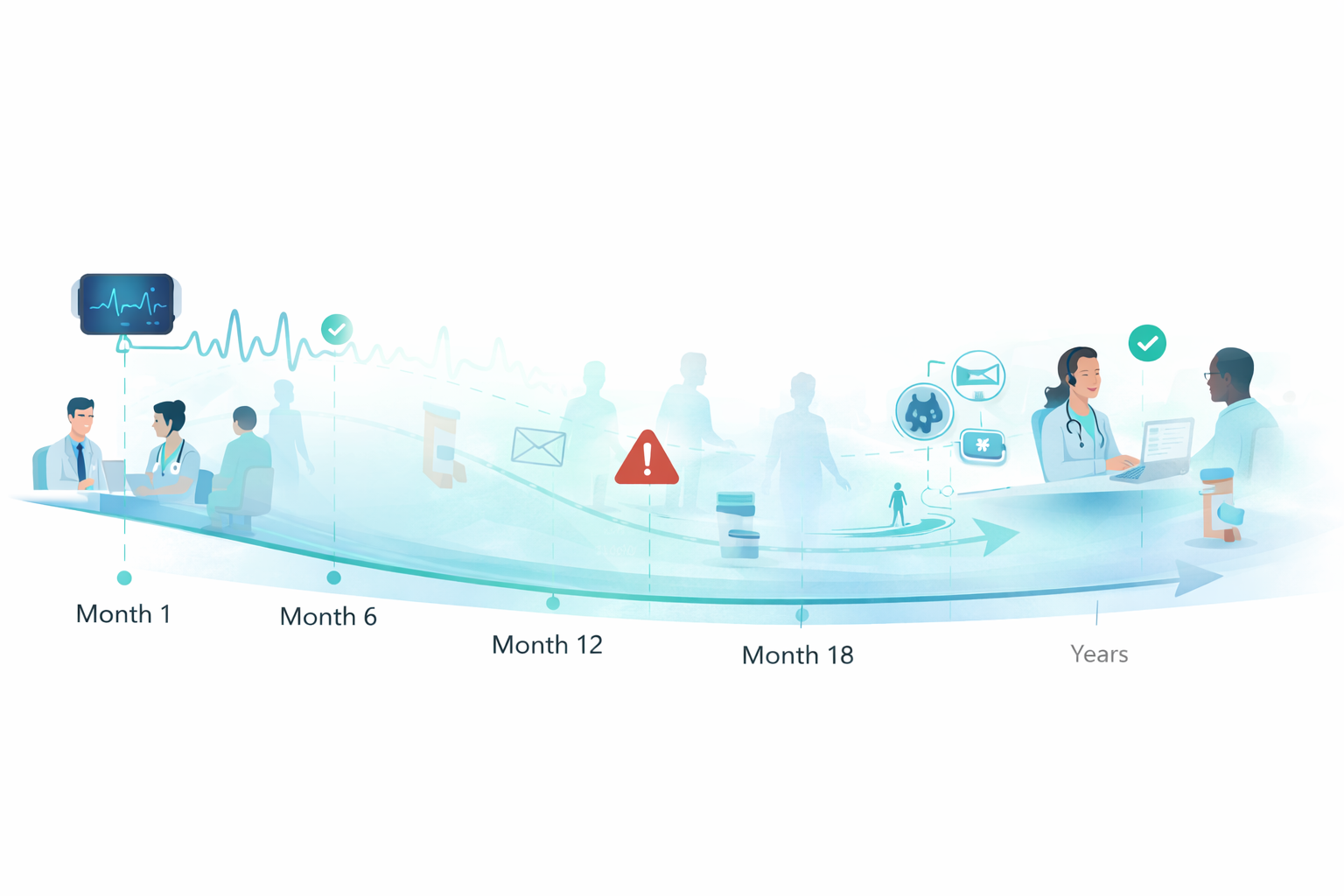
Most operational models are visit-managed. Cardiovascular studies must be continuity-managed — especially in post-market and outcomes programs where the “distance” between touchpoints is measured in months.
Continuity monitoring detects drift early — wear-time gaps, missed routines, delayed uploads, and stale symptom capture.
Concierge-led outreach resolves friction in real time — coaching, troubleshooting, rescheduling support, and device swaps.
Documented interventions: what happened, when it was detected, what actions were taken, and whether continuity recovered.
Protocol-driven routines that remain realistic at Month 12+ — reducing dropout risk without pushing burden back onto sites.
We keep participants engaged when they feel “fine,” keep sites out of the chase, and keep your longitudinal endpoints analyzable.
Why Delve
In cardiovascular research, the endpoint breaks through routine friction: devices aren’t worn, signals degrade, symptom context is missing, and follow-up slips. Delve closes these gaps by owning continuity operations and documenting interventions—continuously, not retrospectively.
Risk: longitudinal wear-time and ECG quality drift between visits; issues are discovered late and fixed expensively.
Risk: “missingness” becomes the story, not the endpoint.
Outcome: higher usable data yield, fewer protocol deviations, lower site burden, stronger retention.
Cardiovascular Evidence Chain
Cardiovascular endpoints depend on longitudinal integrity. Delve closes the loop with QC gates, real-time alerts, and documented interventions when the chain breaks.
How We Engage
Delve leads execution end-to-end with a CV operating model built around continuity, signal quality, and real-time oversight. Specialty functions can be delivered directly or through Delve-led partners as scoped.
Keep your CRO for monitoring/EDC and plug Delve in as the continuity, patient operations, and signal-quality layer. We reduce gaps, improve QC, and provide live risk visibility.
We offload the most time-consuming follow-up—training, troubleshooting, reminders, rescheduling, device swaps—so sites can focus on clinical assessments and enrollment.
Live Metrics (Example)
Example visuals to demonstrate how we monitor wear-time, signal quality, and completion in real time. Your study’s dashboards reflect your protocol, schedules, and site structure.
Markers indicate documented interventions that prevent drift.
Quality gates surface low-confidence segments early, enabling rescue within protocol windows.
Sites with higher friction get proactive concierge support and targeted retraining workflows.
Protocol-to-Analysis
“End-to-end” means ownership with evidence trails—from protocol operationalization and device workflows through continuity rescue, data QC, and analysis readiness.
Step 1
Step 2
Step 3
Step 4
Step 5
Step 6
Step 7
Step 8
Cardiovascular Capabilities
Longitudinal CV signals are only credible if participants actually wear and upload consistently.
ECG and sensor artifacts quietly corrupt endpoints. We enforce QC before data is lost.
Events don’t happen on visit days. Your endpoint needs context without adding work for sites.
Endpoints & Modalities
Delve supports common cardiovascular endpoint strategies by operationalizing routines, quality gates, and evidence trails—so data is interpretable and audit-ready.
Outputs: timestamped episodes, QC flags, event context, intervention logs.
Outputs: BP series, adherence-to-window metrics, missingness reasons.
Outputs: longitudinal activity/sleep features + ePRO completion tracking.
Outputs: symptom trends, completion rates, escalation evidence trails.
Outputs: completion, continuity, and follow-up logs for RWE needs.
Outputs: analysis-ready datasets with handling rules and traceability.
Deliverables
CV studies don’t fail because the endpoint is unknown. They fail because continuity breaks. Delve operationalizes routines, enforces QC, and documents interventions—so your datasets are usable.
If you already have DM/biostats partners, Delve strengthens the operational evidence chain without replacing your stack.
FAQ
Short answers to the questions we hear from Clinical Operations, Digital, and Post-Market teams when planning CV studies.
If your endpoints depend on longitudinal continuity—wearables, ECG, BP routines, symptoms, follow-up—Delve is built to protect that evidence chain. We can lead end-to-end execution or integrate with your CRO to improve compliance and retention.