We’ve blogged about wearables before—what they are; what they can do to advance clinical research; how wearable technology can aid in decentralized clinical trial design; etc.
Now that wearables (e.g., FitBit, Apple Watch) have been widely adopted by consumers and their built-in sensors regularly provide heath data by accelerometer, pedometer, heart rate monitor, etc. And, now that the COVID-19 pandemic has accelerated the rate at which these technology platforms can be adapted and modified to meet the specific needs of clinical trials, there is one glaring question that arises—How do clinical research professionals access and leverage all that data?
According to NIH’s article, Collection and Processing of Data from Wrist Wearable Devices in Heterogeneous and Multiple-User Scenarios, “wearables allow different ways in which data from sensors can be collected by other systems.”
NIH’s article continues to explain:
Proprietary systems can be found as wearable devices, apps for smartphones and computers, and cloud services. These systems are provided and maintained by wearable vendors to collect users’ data, to perform analytics and to provide data and analytic results to users and to authorized third-parties. Third-party systems such as services, apps for smartphones and wearables, and programs for computers can be developed and maintained by external entities to provide specific functionalities. Each one of these components is intended to perform some specific functions:
- Data collection.
- Data transfer.
- Permanent data storage.
- Data analysis.
Basically, wearable devices have and will continue to revolutionize how we collect a patient’s data. But once all that data has been mined, how do clinical research professional transfer that data? Store that data? Analyze that data?
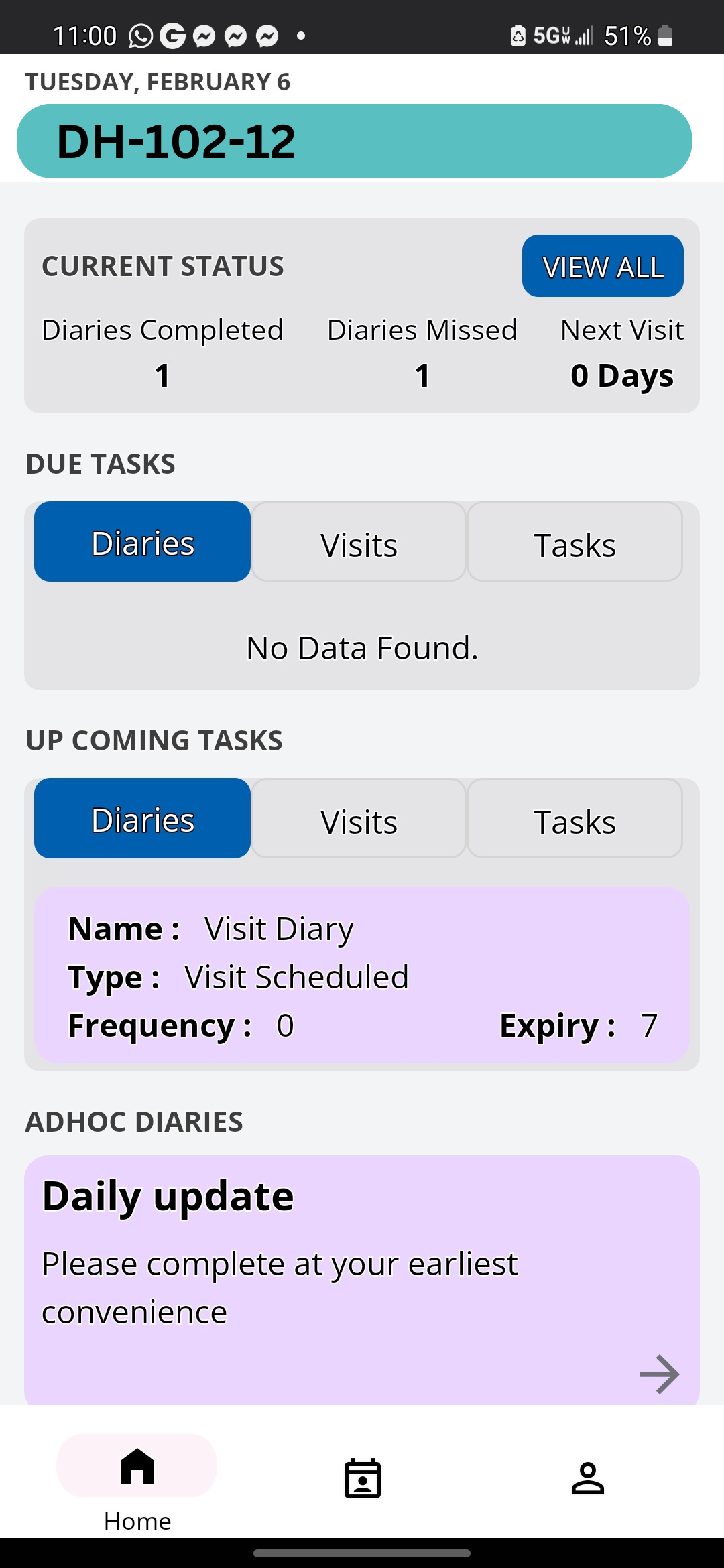
Delve Health’s Clinical StudyPal is the answer to all your questions. Our software solution uses artificial intelligence (AI) technology and configurations to help you access the data easily and more efficiently than ever before.
For example, a wearable equipped with the Clinical StudyPal app allows a patient to both actively and passively have their data collected (e.g., eConsent, ePRO/eCOA, eDiaries, and other health data such as heart rate, etc.). Data connectivity allows for data access. Our AI engine reads the data inputted and learns when the platform should “nudge” the patient.
These notifications are crucial in pairing AI and wearable medical devices in order to improve patient compliance. For example, let’s say it has been a few days since the wearable has reported any data at all. Chances are, the patient has forgotten to wear the device. Clinical StudyPal’s AI will reach out via a text message to remind them to wear their wrist device. Once worn, the platform can remind the patient to fill out their eDiaries, take their heart rate, or exercise to ensure patient compliance.
The platform also provides clinical researchers with the option to view data for reporting purposes. Users can apply filters to ensure they can locate and analyze their specific data points for reporting purposes. Clinical StudyPal can even be uniquely tailored to allow the end user to download reports based on their specific parameters.
Leveraging wearable technologies, along with Delve’s Clinical StudyPal platform, lends itself to one specifically large benefit—an increase in decentralized trial designs, which in turn leads to the benefit of increasing diversity within clinical trials.
In a decentralized trial, data comes from multiple sources and we need to be ready to integrate, validate and collect all appropriate data across various digital endpoints—Delve Health can help.