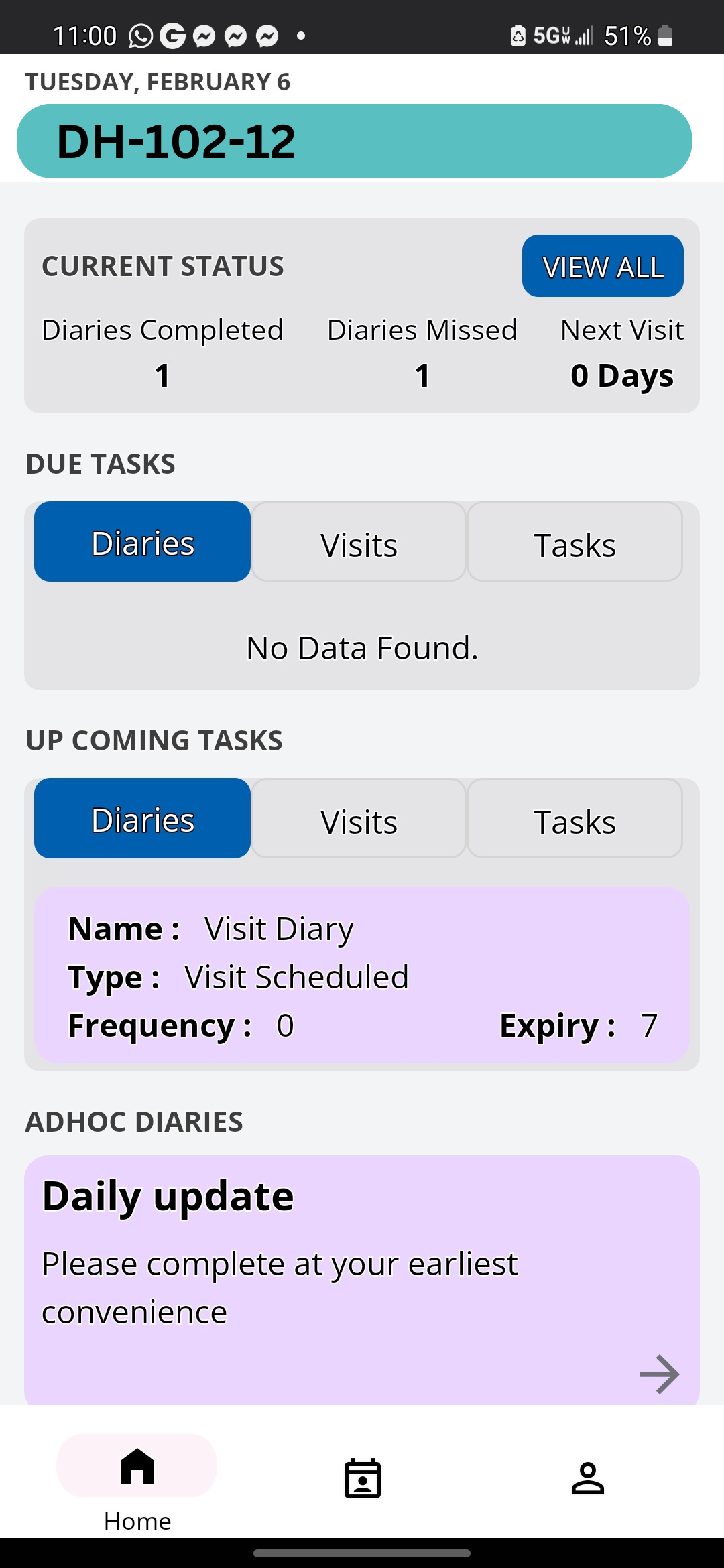
At Delve Health, we took extensive measures to develop a scalable eConsent solution that can be integrated with various solutions to streamline the data flow.
Below are the core elements we implemented to ensure scalability and flexibility
- Flexible workflows: Flexibility to accommodate multiple prescreening, screening, and re-consent, all with a simple interface to help users easily manage the eConsent.
- Version Control: In order to allow for Protocol amendments, Clinical StudyPal allows for multiple versions.
- Global Access: Allowed for different consents to be developed for different countries. An eConsent can be developed at a site level, country level, global level.
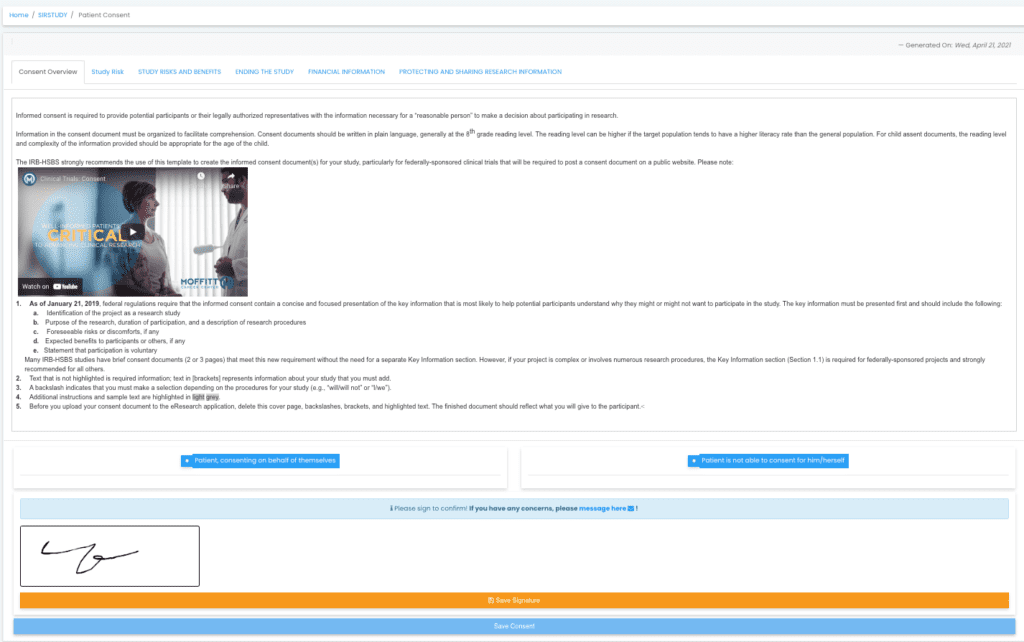
- Multimedia libraries: Informed consent forms have become longer and more complex. Patients and caregivers may be dealing with illnesses and high-anxiety situations, which make reading ICFs difficult. Without our eConsent design form, the user can easily upload multimedia (video/images) to simplify the consumption and comprehension of the data.
- Socially distant signatures: Allowing individuals to conduct a video signature or utilize their phone to sign and upload their ID.
- Remote monitoring: Allowing the study team to easily review all the consent information.
- Site Review: Investigators can simply review the patient consent remotely, and sign/approve the consent information electronically.
Robust Technological Infrastructure
Delve Health’s eConsent solution leverages the Google Cloud infrastructure. Google Store creates a secure, redundant, and scalable platform for the application to run on. Google HIPAA compliant infrastructure also ensures that personally identifiable information data is not moved across borders from where it is collected, a critical component of privacy regulation compliance. The system also supports single sign-on capabilities, allowing site users to effortlessly move between Complete Consent, electronic data capture, IRT, and any other technologies using the same service. Single sign-on solutions are 21 Code of Federal Regulations Part 11 compliant and increase efficiency and reduce frustration without increasing risk. Click here for a 30 min demo.